Learn more about the NEO-Empower clinical research study
If you have recently been diagnosed with NSCLC, please know that you are not alone in your medical journey. Lung cancer is the second most common cancer diagnosed in both men and women in the United States.
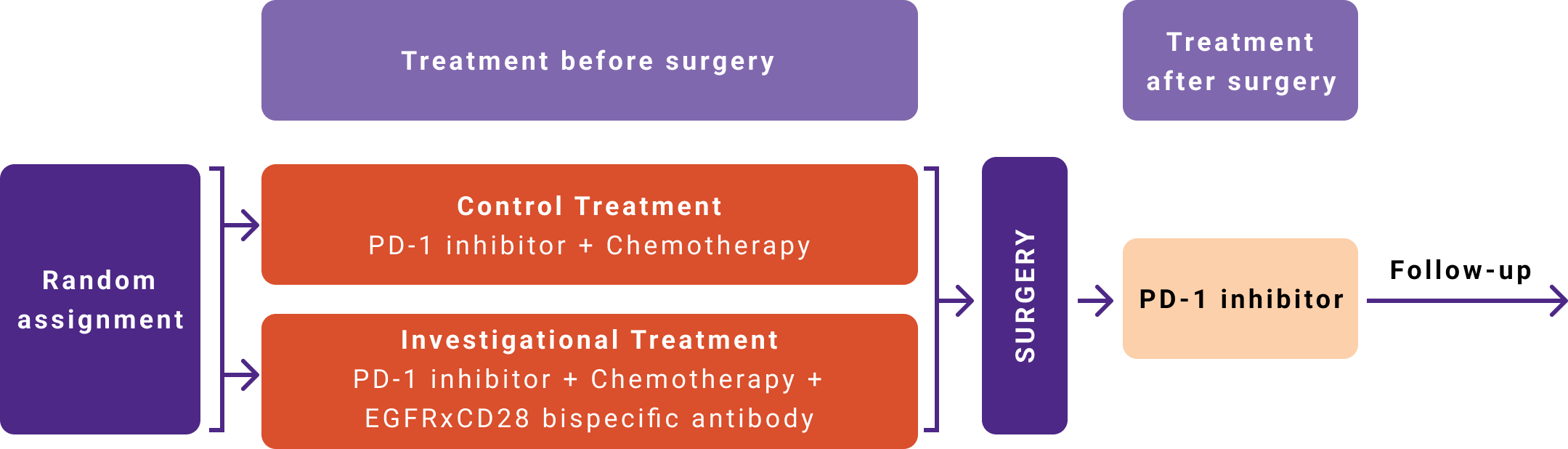
We are working on developing potential treatments for lung cancer that is diagnosed at an early stage and can be removed by surgery. This includes some stage II and III lung cancers. NEO-Empower is a phase 2 clinical research study that aims to test the efficacy and safety of adding an additional investigational anti-cancer drug to the combination of chemotherapy and a PD-1 inhibitor in patients with early-stage NSCLC for whom surgery is planned.
If you are eligible, you will undergo perioperative therapy (treatment both before and after surgical removal of the lung cancer).
What are the study treatments?
Chemotherapy is a standard treatment for early-stage NSCLC. The other study treatments are called “investigational” because they have not been approved to treat early-stage NSCLC.
The investigational PD-1 inhibitor is an immunotherapy that has been approved by the U.S. Food and Drug Administration (FDA) and several other countries for the treatment of advanced NSCLC but has not been approved for the treatment of early-stage NSCLC. This drug is a monoclonal antibody that blocks the programmed death receptor 1 (PD-1), a cell receptor on immune cells. This drug works in a similar way to other PD-1 inhibitors that have been approved for the treatment of early-stage NSCLC in combination with chemotherapy.
The other investigational drug is an EGFRxCD28 bispecific antibody that binds to the proteins EGFR (epidermal growth factor receptor) that is expressed on lung cancer cells and CD28 (cluster of differentiation 28) that is expressed on T-cells. The EGFRxCD28 bispecific antibody may help promote stronger anti-tumor immune system responses, which means it could help the immune system fight lung cancer more effectively.
Once the study doctor confirms that you qualify, you will be randomly (by chance) assigned to one of several possible treatment groups: the control treatment group or one of the investigational treatment groups that is active at the time you join the study. You will have an equal chance of being placed into each group. The control treatment group consists of chemotherapy plus the PD-1 inhibitor and is intended to reflect the usual or standard treatment for early-stage NSCLC. The first investigational treatment group consists of chemotherapy plus the PD-1 inhibitor plus the EGFRxCD28 bispecific antibody.
The treatment period in both the control and investigational treatment groups will consist of pre-surgery and post-surgery treatment periods separated by surgery. In both the control and investigational treatment groups, surgery will be followed by additional treatment with the PD-1 inhibitor alone. All patients, whether assigned to the control or the investigational treatment groups, receive the same treatment after surgery.
If the surgeon is unable to remove the tumor successfully, then your participation in the study might stop. If that happens, your doctor will discuss the appropriate next steps with you.

What can I expect if I participate?
Study participation is 100% voluntary (your choice). You will receive information on what to expect, as well as your roles and responsibilities if you join the NEO-Empower study. You may leave the study at any time without affecting your regular health care.
If you join the study, there are certain things you will be expected to do. This includes attending study visits and undergoing certain assessments and procedures, including biopsies, imaging scans, and blood draws. The length of your participation in this study will vary depending upon your response to the study drugs, whether the cancer comes back, and side effects you experience from the study drugs.
Before you start treatment, there will be a period of up to 4 weeks where you may be asked to undergo tests to make sure that you qualify for the study. Following this, the treatment period (including the pre-surgery treatment, surgery itself, and post-surgery treatment) may last for approximately 12 months. You will need to return to the clinic 30 and 90 days after the last dose of the study drugs to check for side effects, and you will then continue to be monitored to check for recurrence of your cancer. After the 90-day follow-up, you may also be contacted by telephone about the status of your health every 3 months.
There will be no cost to you for the study drugs, visits, tests, or supplies that are required for the study. Reasonable travel and food costs will be reimbursed to you. The study team will discuss all of this with you.
How can I take part?
If you wish to take part in NEO-Empower, please speak with your oncologist to see if you may be eligible.
You may be eligible if you:
- Are ≥18 years of age
- Have been diagnosed with early-stage (stage II to IIIB) NSCLC
- Have not yet started treatment for the lung cancer
- Are eligible to remove your NSCLC tumor by surgery
There are other requirements to participate. A full medical check-up will be done to see if you can join this study. If you have other questions, share this website with your oncologist to discuss this study in additional detail. You can also find more information about the study and participating sites using the registries listed below.
What else should I know?
As with all medicines, there are possible risks when taking the investigational treatment. If you qualify and choose to participate, you will be provided with an Informed Consent Form that explains any possible risks and side effects. It is also possible that the study treatments may affect you in unknown ways. Your health and safety are our top priorities and will be closely monitored throughout your participation.
There is no guarantee that you will receive a medical benefit from participating in this study. Your condition may get better, stay the same, or may even get worse. You are free to withdraw from the study for any reason and at any time.